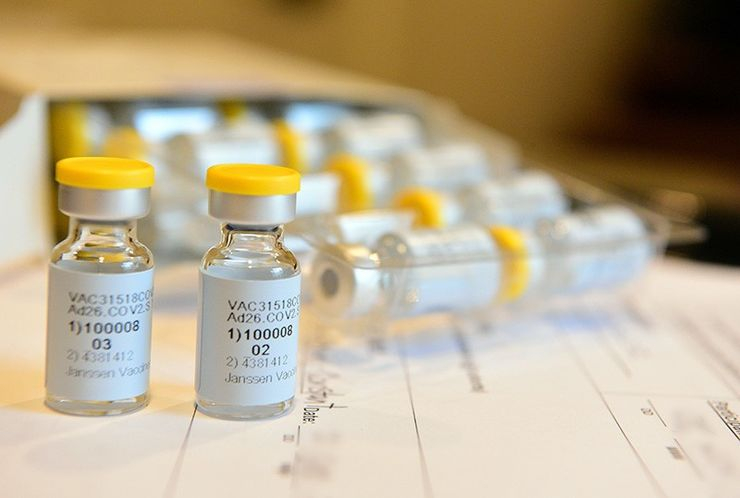
A coronavirus vaccine from a unit of Johnson & Johnson is set to move to the third phase of clinical trials in the U.K. on Monday that will test the safety and effectiveness of the shot.
The testing round by Janssen Pharmaceutical Companies will include 6,000 volunteers and take place across 17 sites, according to a statement. The vaccine is the third candidate to be tested in the U.K., alongside one from an AstraZeneca in partnership with the University of Oxford and another from Novavax Inc., it said.
Progress toward creating vaccines against Covid-19 has accelerated this month, with Pfizer Inc. and BioNTech SE saying their shot prevented more than 90% of symptomatic infections in a trial. Still, hurdles for production and development remain before vaccines could reach widespread use.
Recent data indicate “we could be on the cusp of the first major breakthrough since the pandemic began,” U.K. Business Secretary Alok Sharma said. “While we are optimistic with the progress being made, there are no guarantees and it is possible there will be no one-size-fits-all vaccine.”
If the vaccine from New Jersey-based Janssen proves safe and effective, 30 million doses could be available in the U.K. by mid-2021, according to the release. The government’s Vaccine Taskforce is partially funding the effort.
The government also announced that it will open two new “megalabs” early next year, which will together increase daily testing capacity by 600,000. The labs will be used for Covid-19, as well as testing for other critical illnesses such as cancer, according to a statement.
The U.K. recently became the first country in Europe to record more than 50,000 deaths from the virus, and England is under lockdown restrictions until early December due to a surge in cases.